Consider the case of Minnie Halsted: A young woman in Albany, New York, who started severely hemorrhaging after giving birth in 1881. She was saved by her brother William Stewart Halsted, the famed New York City (and later Baltimore) surgeon sometimes called the father of modern surgery.
What Minnie’s brother did was to draw his own blood through a needle and infuse it directly into her vein—analogous to the transfusions that are performed routinely today in relative safety—but that was orders of magnitude more risky 140 years ago. Doctors in those days knew that transfusion was dangerous, but the phenomenon of blood incompatibility would not be discovered for another two decades, when Viennese researcher Karl Landsteiner first described what we now call the ABO blood groups.
Knowing that his sister’s chances of survival were not good either way, Halsted’s decision to donate blood came down to a risk-benefit analysis—less informed, but otherwise not so different from decisions surrounding modern transfusions, all of which carry some degree of risk for complications, such as lung damage and overloading of the circulatory system. In 1881, however, Halsted had not even the foggiest notion of the complication that transfusion medicine experts today routinely prevent and manage through careful blood-type matching. Hemolysis, or the breakup of red blood cells, can be fatal if it happens in the context of a mismatched transfusion.
Minnie recovered. Her brother saved her life— but only through sheer luck. Her blood type and her brother’s blood type just happened to match in a way that made him an appropriate donor for her.
Today, rather than whole blood, patients are infused with individual, specifically needed blood components, such as packed red blood cells (pRBCs), plasma, or platelets, and the blood must be matched by type. In the future, however, perhaps by mid-century, matching may not be required, because of an emerging innovation: universal blood. The idea is not new. In fact, clinical trials at the dawn of the current century tested the prospect in a limited form—type O red blood cells were produced from B red blood cells utilizing an enzyme from green coffee beans. The idea has emerged again now, because of advances in regenerative biology. Using hematopoietic stem cells and with aspirations for clinical trials sometime in the current decade, a handful of startup companies are generating red blood cells that would both qualify as universal, meaning appropriate for most recipients, and, importantly, that would be commercially viable.
“Universal red blood cells will alleviate blood shortages (especially type O-negative) and simplify blood supply and donor recruitment logistics, especially during pandemics and other natural disasters, where donor centers are stressed trying to collect and arrange for blood products,” says Gagan Mathur, a professor of clinical pathology at the University of Southern California’s Keck School of Medicine, who specializes in transfusion medicine. “Such a product would obviate incompatibility, alloimmunization, and hemolytic transfusion reactions resulting from them, especially in patients that require repeated transfusions.”
The molecular basis of blood compatibility
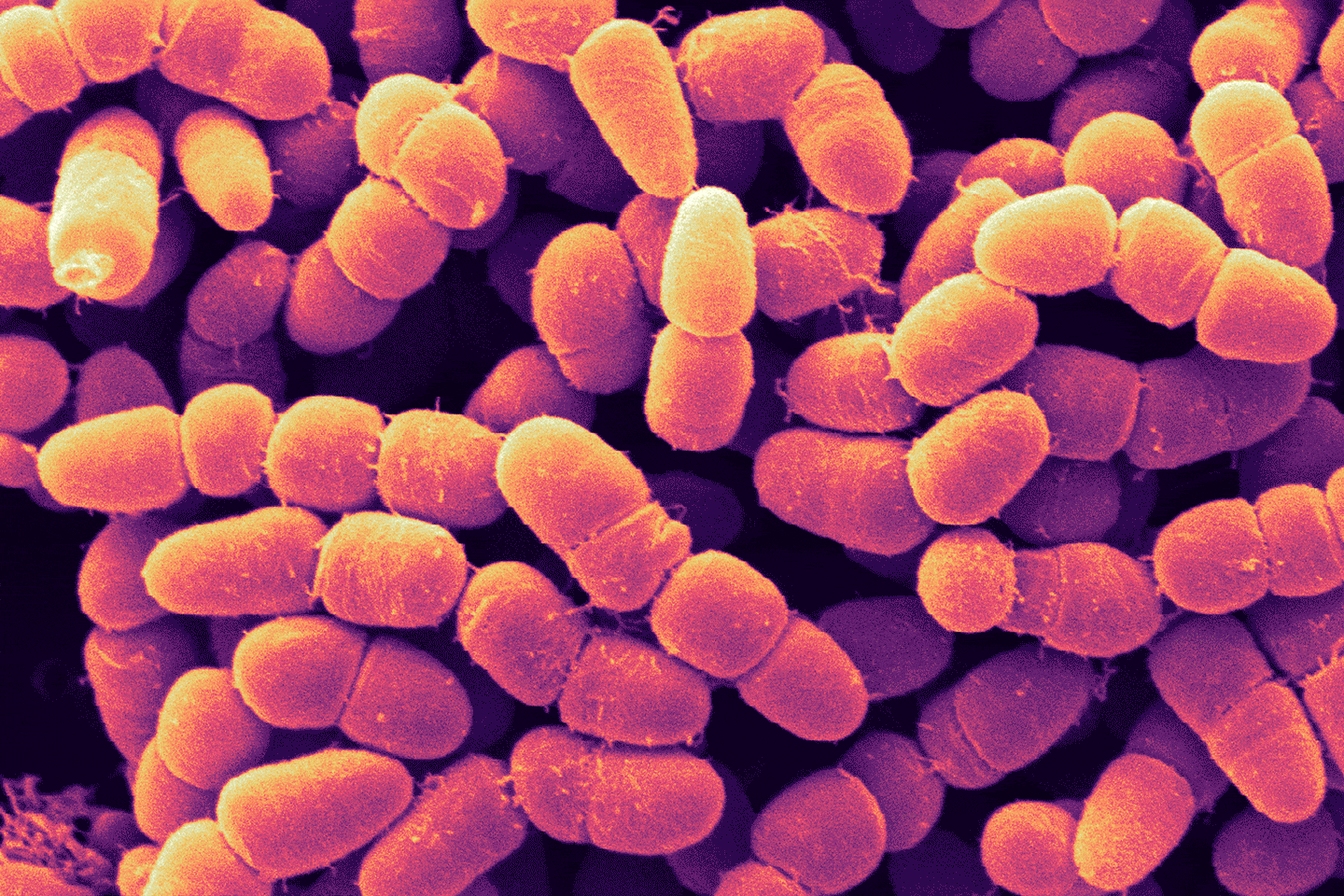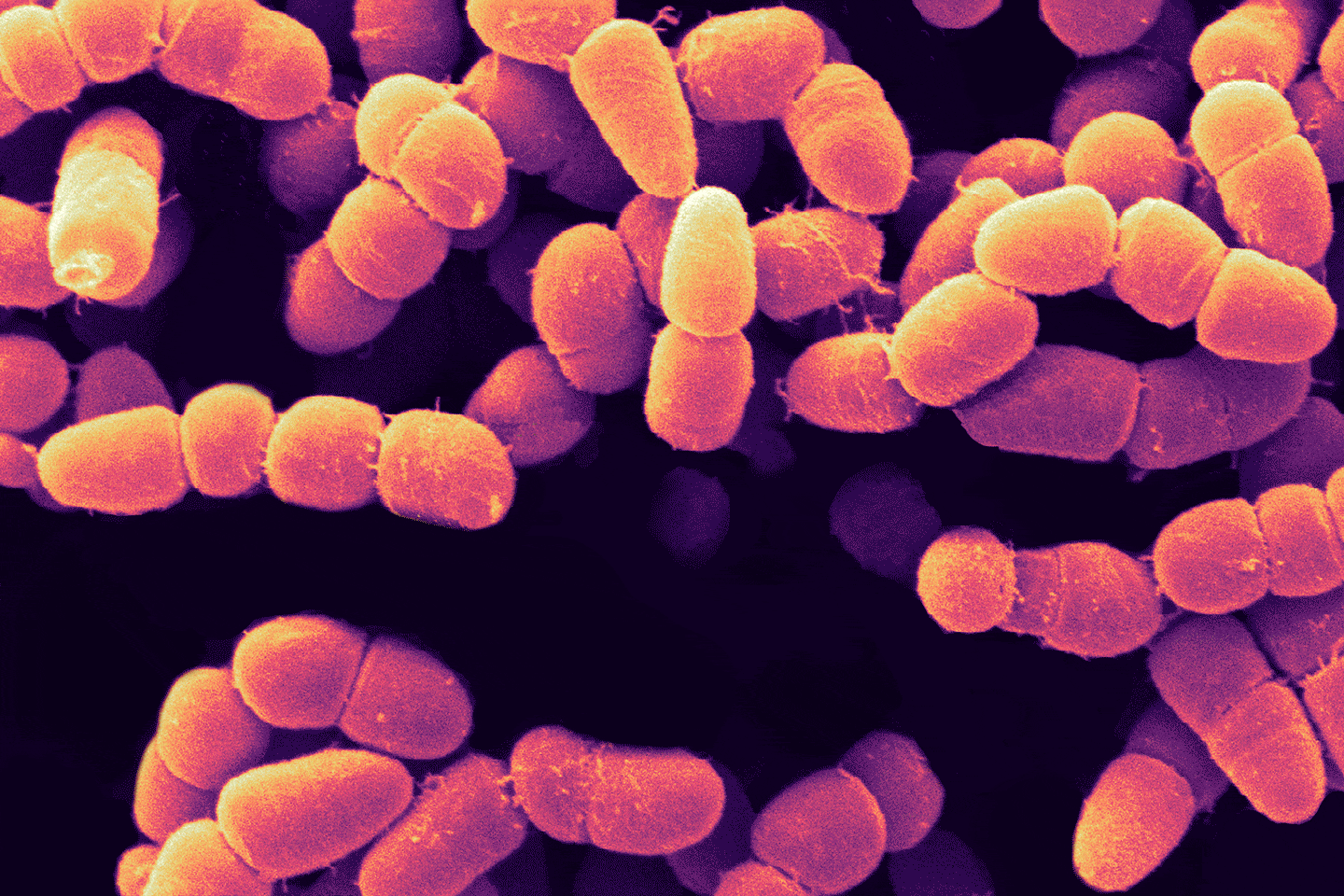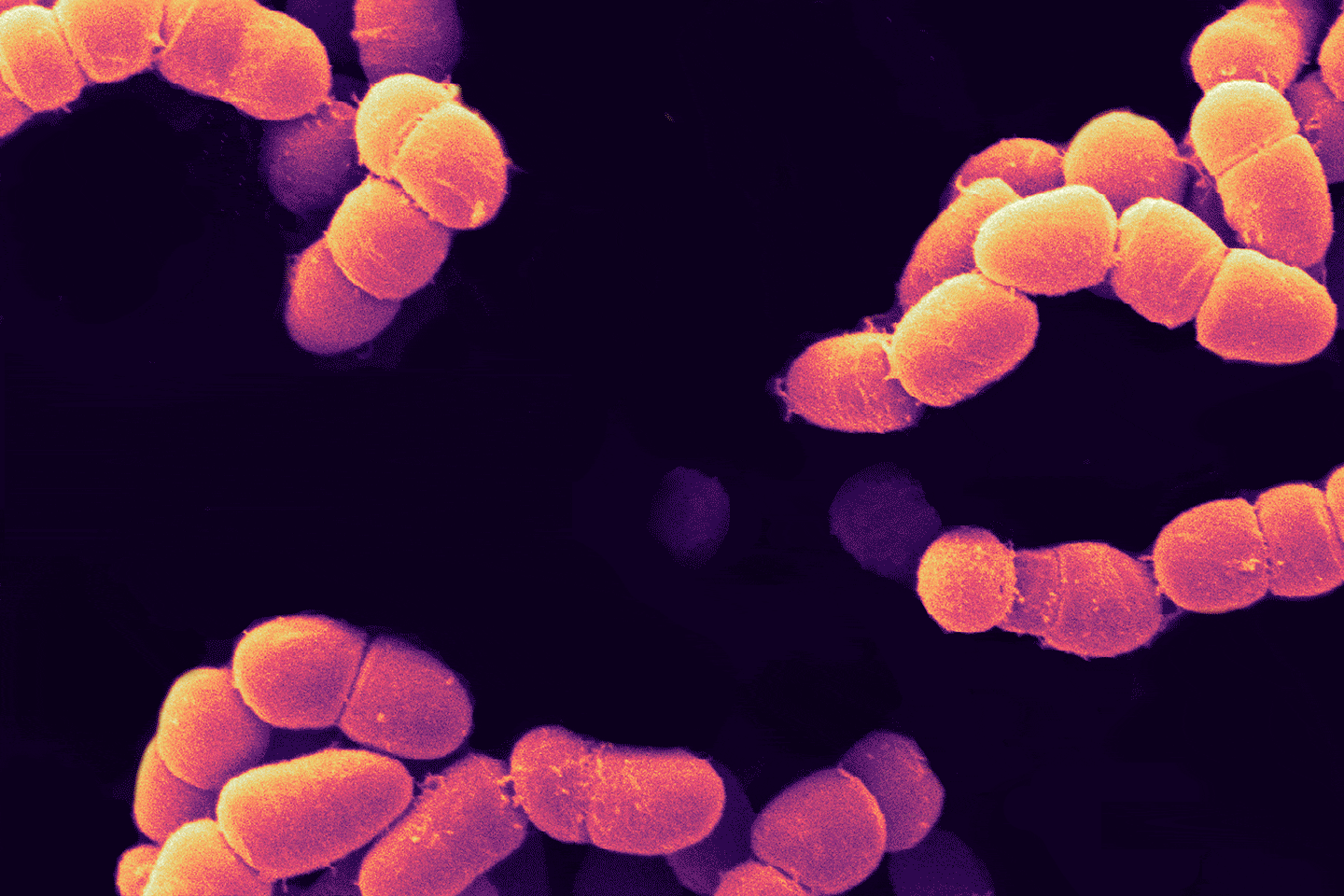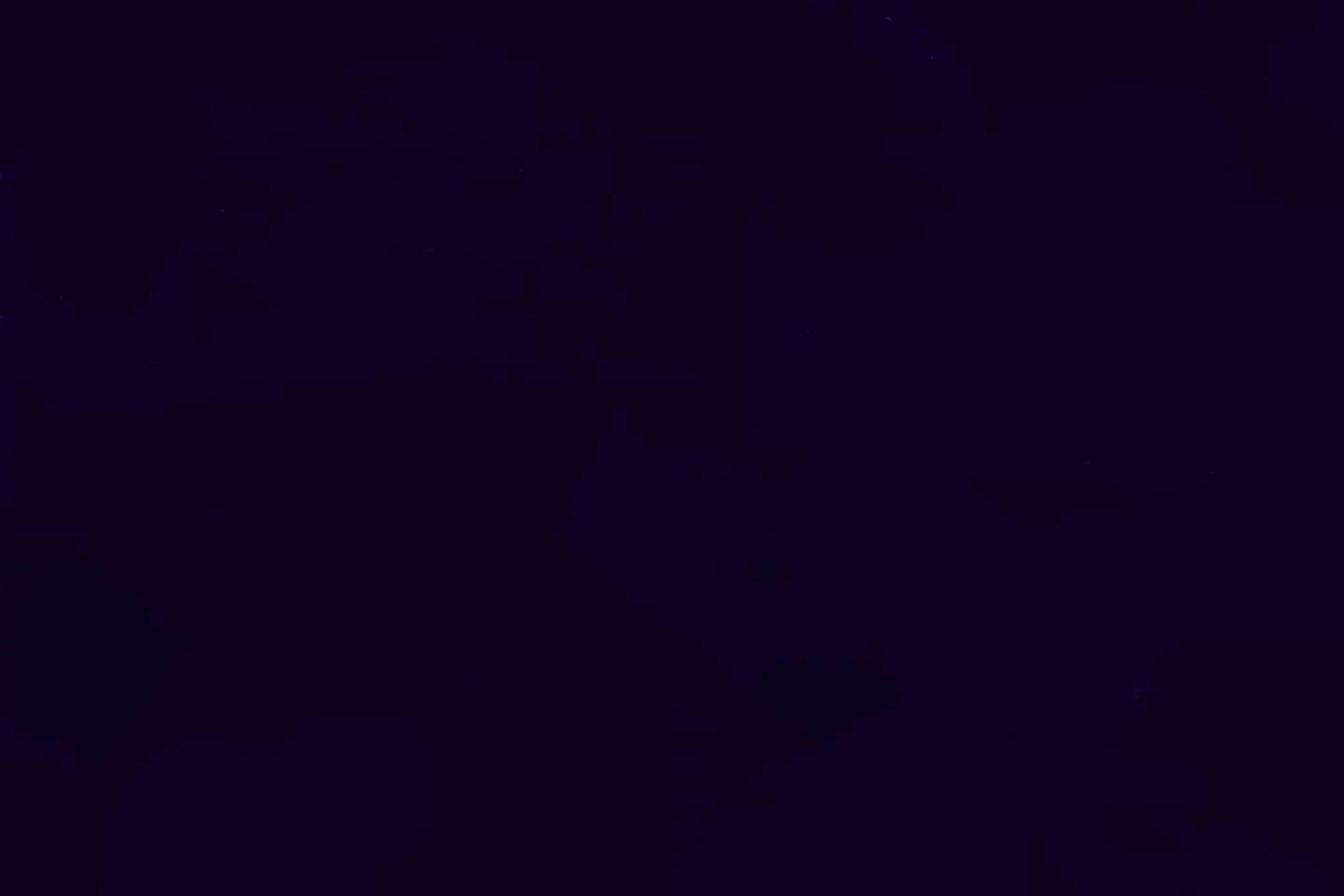
Incompatibility, alloimmunization, and hemolysis—put simply, what Mathur means is that, when presented with donor red blood cells that it does not recognize as self (due to the presence of certain molecules on the surfaces of the donor cells) the immune system attacks the donated cells, breaking them apart, leading to a cascade of events that can be fatal. Considering the ABO blood groups, the most familiar blood group system, people with type O-negative blood are called universal donors because they are able to donate blood not only to people who have O-negative blood as they do—but also to people who have O-positive, A-positive, A-negative, B-positive, B-negative, AB-positive, and AB-negative blood types.
Other blood types are more restrictive—some extremely so. Type B-negative, for example, can be given to people who are B positive or negative, as well as to people with type AB blood (positive or negative). But the compatibility ends there. People with blood type O or B will suffer a severe immune response if given blood of type A, whereas people of type O or type A will suffer a severe immune response if given type B. Type AB-positive is the least versatile, and it can be given only to people who are also AB-positive.
In the 1980s scientists found that an enzyme from green coffee beans could be utilized to create type O red blood cells with better efficiency.
As noted above, the reason for this selectivity is the presence of molecules on the red blood cell surface. Designated A, B, and H, these surface molecules are called antigens, because two of them—molecule A and molecule B—can trigger an immune response in blood recipients whose own blood cells do not have them, and so they are seen not as self, but as foreign. As for molecule H, although called an antigen, it does not provoke an immune attack in anybody. How then do these antigens relate to the blood types? Type A red blood cells carry the A antigen, type B carry the B antigen, and type AB cells carry both A and B. As for type O, these cells carry only the H molecule, which is why the cells can be given to recipients of any ABO type. Zooming in on these antigens we find that the differences are surprisingly subtle: An A antigen is simply an H antigen with one extra sugar at the tip, whereas a B antigen also has an extra sugar, although it’s a different sugar than the one present on the A.
It’s actually more complicated than that, as transfusions are affected by the presence or absence of the Rh factor (the antigen responsible for the “positive” or “negative” following the A, B, O, or AB of the blood type) and by other blood antigens, some with unlikely names like Duffy, Kidd, and Kell, and those are just the tip of the iceberg. In reality, there are dozens of different blood group systems, leading to several hundred different blood types, but the good news is that not all of the potential mismatching between types is clinically significant, especially in patients who are not receiving frequent transfusions.
Changing blood types
About midway through the last century, scientists began noticing that, on rare occasions, a person’s blood could change its type spontaneously. This raised the possibility of deliberately changing blood in the laboratory—if not to produce a truly universal blood lacking all of those antigens, then at least to remove some of the most problematic antigens.
We now know that these rare blood type changes in the ABO system happen naturally because of enzymes that can snip off, or replace the terminal sugar. Usually, these enzymes come from bacteria, but in the 1980s scientists found that an enzyme from green coffee beans could be utilized to create type O red blood cells with better efficiency, but with one major drawback. This particular enzyme must have type B red blood cells as starting material. Present in only 7 percent of people, type B is more rare than type A, and thus has limited potential as starting material. Moreover, available enzymes can’t remove the Rh factor, and so creating type O-negative blood with this method requires type B-negative, which is even more rare, comprising just 2 percent of human blood. Even so, for proof of concept, O red blood cells, made enzymatically from B red blood cells, reached clinical trials in the early 2000s, where they proved to be not perfect, but adequate, meaning that there were traces of B antigen, but not enough to cause a problem.
While an enzyme pair for making type O from type A has also been discovered, it works less efficiently than the B antigen enzyme, so the product has not been tested clinically. Moreover, the lack of an enzymatic process for neutralizing the Rh factor means that O-negative cannot be made from O-positive, which circulates through the veins of 38 percent of all people.
The challenge of scaling up red blood cell production to commercial levels is not unlike that faced by the cultivated meat industry.
In the last few years, the attention in the field has shifted away from the enzymatic approach to regenerative biology, using stem cells to generate the needed universal red blood cells. While a multitude of companies are working on stem cell-based therapeutics for a range of blood diseases, very few are working on making red blood cells themselves. proto.life spoke with the CEO of one of the few companies that is.
“While stem cell approaches of the past have produced red blood cells, only in small quantities and at a cost too high to allow commercialization. Current stem cell cultivation technologies will soon enable red blood cell production at great mass,” notes Ari Gargir, the CEO and founder of Red C Biotech, an Israeli company that is using stem cells to produce a commercially viable blood product that will be O-negative and also negative with respect to the Kelly, Duffy, and Kidd antigens.
According to Gargir, the challenge of scaling up red blood cell production to commercial levels is not unlike that faced by the cultivated meat industry, which also relies on taking stem cells, differentiating them, and growing massive amounts of product in industrial fermenters. But he predicts his industry will be helped by a price point for blood products that far exceeds faux meat. “While the cultured meat industry will produce 1 kilogram of meat for $5, the red blood cell cultivation will need to produce, at pharmaceutical standards, just 100g of [cells] for $50.”
While universal red blood cells seems to be where things are headed in transfusion medicine in the decades to come, this does not mean that some other alternatives haven’t been considered, one notable alternative being synthetic blood, designed around polymers and other molecules designed to dissolve in blood and carry oxygen, but without being packed into cells. “Synthetic blood substitutes have been shown to be toxic,” notes Gargir. “So far, these attempts have failed and have not been commercialized.”
Whether for those with hemophilia, thalassemia, sickle cell disease, or other conditions requiring frequent transfusions throughout life, or for those with traumatic blood loss injuries or other conditions requiring large transfusions, universal blood beckons to advance medicine one more notch ahead.